All Locked Up
A new remediation tool uses a chemical compound to immobilize a range of metals that are common groundwater contaminants.
- By Bryan W. Vigue, Anna Willett, Stephen Koenigsberg
- Nov 01, 2003
Accelerated Natural Attenuation
Accelerated natural attenuation is a method of in situ groundwater remediation that has found a niche between expensive, mechanical pump-and-treat protocols, injection/extraction and monitored natural attenuation, which is burdened with a "do nothing" reputation. Accelerating the attenuation rates of organic contaminants like fuels, solvents and pesticides in aquifers via the injection of substrates has become a widely accepted, inexpensive and successful groundwater remediation approach that has been proven on thousands of sites worldwide. Organics are not the only contaminants amenable to accelerated natural attenuation; the remediation of dissolved metals can also be engineered to increase attenuation rates and decrease time to site closure. More specifically, a new product, Metals Remediation Compound (MRCTM) from Regenesis of San Clemente, Calif., is now commercially available for accelerating in situ metals cleanup in groundwater systems. Like the process of in situ bioremediation of organics, in situ metals remediation promises to be an effective, low-cost and long-term solution for the restoration of metals contaminated aquifers.
Extent of Metals Contamination Problem
Metal contaminants are present at many sites undergoing remediation in the United States. For example, soil and groundwater are contaminated with metals at:
- 65 percent of 944 National Priorities List (NPL) sites1,2
- 46 percent of 214 sites regulated under
the Resource Conservation and Recovery Act (RCRA) 2,3
- 69 percent of 3,212 U.S. Department of Defense (DOD) sites 2,4
The most common metal contaminants at all U.S. sites are lead (Pb), chromium (Cr), arsenic (As), zinc (Zn), cadmium (Cd), copper (Cu), mercury (Hg) and mixtures thereof.5 Most of these metals have documented negative human health effects at low microgram-per-liter concentrations. Contamination by radioactive elements is an additional and extremely important concern. Uranium (U) is present in soil and groundwater at 70 percent of U.S. Department of Energy sites where nuclear weapons and reactor activities were conducted.6 Uranium is also found at mining and ore processing sites throughout the United States.
The most common sources of metals contamination in groundwater are leachate from landfills, mine tailings piles and abandoned mines; sewage sludge or liquid sewage; and spills, leaks and land disposal of wastes at industrial facilities.7 Industries that typically use metals and are potential sources of contamination are plating (Cr and Cd), battery manufacturing nickel (Ni), Cd and Pb, wood preserving (Cu, Cr, and As), chemical manufacturing (Cu, Zn, Pb and Hg) and smelting (Cu, Zn, Pb and Hg).8
Metals Remediation Processes
Metals remediation consists of ex situ (above ground) and in situ (subsurface) processes. When applicable, in situ processes are often more cost-effective and easier to implement than ex situ processes since in-ground treatment eliminates excavation, disposal and/or pump-and-treat activities. One primary method of in situ metals remediation is metals immobilization. Immobilization refers to the process of transferring aqueous phase, highly mobile metals to a solid, stable phase that becomes part of the soil. This phase transfer prevents continued migration of contaminated metals plumes and can be a permanent solution depending on the metal and site-specific geochemistry. The most common mechanisms of in situ metals immobilization are metals adsorption to soil particles or precipitation of metal solids that are chemically fixed to soil particles. Both of these mechanisms can permanently remove metals from the aqueous phase, restoring the aquifer and the desired usability of the water.
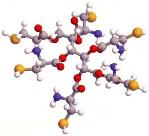
Metals Remediation Compound
MRC Description
MRC is a slow-release, non-toxic product that removes dissolved metals from groundwater via in situ immobilization. The active compound in MRC irreversibly complexes with metal ions, and these complexes adsorb strongly to soil, eventually precipitating as insoluble sulfide minerals. For many metals (e.g. Pb, As, Zn, Cd, Cu, Hg and Ni), sulfide solids are extremely stable and recalcitrant to re-dissolution and the sequesterization of metals as sulfide solids is an accepted method of groundwater detoxification. 9, 7, 10
MRC's Active Compound
MRC's active compound is an ester of cysteine (a sulfur-containing amino acid) and a carbon backbone molecule, like glycerol or sorbitol. A ball-and-stick diagram of sorbitol hexacysteinate (SHC), one version of the active compound, is shown in the image above (Figure 1). Note that cysteine is a naturally produced, organic molecule that is formed in many bacteria, plants and animals in response to the presence of metals. 11
Cysteine is a component of several large, biological macromolecules that are used for metabolic functions, such as intracellular fixation of trace nutrient elements like copper and zinc, regulation of metal transport to metabolic centers, and neutralization of the toxic effects of metals like cadmium and mercury. 12 Thus, a cysteine-based product such as MRC is well suited for in situ metals immobilization, since it has a strong affinity for many metal contaminants and does not drastically alter the chemical and physical properties of the subsurface. MRC merely takes advantage of and improves upon a naturally occurring metals sequestration reaction.
Longevity in the Subsurface
The SHC in MRC is embedded in a polylactate matrix that provides a carbon source and electron donor for subsurface bacteria. This combination of materials makes MRC a viscous but injectable material that slowly releases the cysteine ester to a contaminated aquifer via hydrolysis by water or enzymatic action by microbes. One benefit of MRC's slow-release property is its longevity of 12 months to 18 months in an aquifer. A single injection of MRC can provide metals remediation activity for over a year, allowing an effective approach to metals immobilization.
Contaminants Treated
To broadly meet the apparent need for the remediation of a suite of metals, MRC is designed to precipitate a wide range of dissolved metal ions, including heavy metals
(Cr, Cu, Ni, Zn, Cd, Hg, Pb and others), semi-metals As and selenium (Se) and actinides (U and others).
Additionally, MRC's polylactate base provides a substrate for indigenous bacteria to reductively dechlorinate chlorinated solvents. Therefore, MRC can be applied to accelerate the bioremediation of mixed metals and chlorinated solvents plumes. The use of one product to treat both types of contamination conserves time and money, as dual treatment technologies need not be implemented.
Detailed Chemistry
The organosulfur moieties in MRC interact with metals either to complex them (e.g. Cd and Hg) or to reduce them and complex them sequentially (e.g Cu and As).13 The organic portion of MRC is biodegradable, and biodegradation leaves a metal sulfide solid for metals that form stable sulfide solids (e.g. Cu, Ni, Zn, Cd, Hg, Pb, As and others). The long-term fate of such metals will be the same as the corresponding metal sulfide minerals e.g. NiS(s), PbS(s), HgS(s), etc. and is, under many geochemical conditions, incorporated into the soil matrix as a sulfide solid.
The organosulfur moiety in MRC will also act as a reducing agent for metals that precipitate as oxides and hydroxides under reducing conditions. For example, chromium (Cr) is reduced from Cr(VI) to Cr(III) and then precipitates as a hydroxide solid, chromite Cr(OH)3(s). Uranium (U) is reduced from a soluble U(+6) oxidation state to the U(+4) oxidation state, which precipitates as an oxide, uraninite UO2(s).
Laboratory Soil Column Experiments
Description of Experiments
MRC was tested for immobilization of dissolved arsenate As(V), chromate Cr(VI) and copper mixtures in simulated aquifer experiments. This mixture represents a common wood-preserving blend. A second study was performed with cadmium, mercury and lead, which represent common heavy metal contaminants.
Experiment Set-up
The experimental system consisted of an Aquifer Simulation Vessel (ASV), which has successfully been used for other bioremediation studies with various groundwater contaminants.14 The ASV is a 6-foot long tube with a 6-inch diameter that is packed with soil for bioremediation testing. It has sampling and injection ports along the bottom at various intervals, and it can be run either as a flow-through system or saturated with water, but with no flow.
In these experiments, MRC was injected into a static ASV that was saturated with contaminated water. For the wood preservative mixture (As, Cr and Cu) study, the ASV was loaded (pore spaces filled) with As:Cr:Cu at 26 :15 :4 mg/L (milligrams per liter), respectively. For the heavy metal study (Cd, Hg and Pb), the ASV was loaded multiple times with each metal at concentrations in the 20 mg/L to 100 mg/L range (details given later). A mass of pure SHC, MRC's active compound, was input to the influent end of the ASV, and the flow was turned off. The ASV was periodically sampled for dissolved metal concentrations at three to five horizontal positions along the length of the vessel.
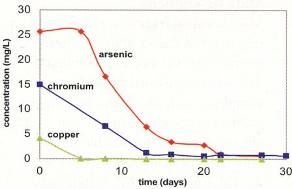
Results for Arsenic, Chromium and Copper
The half lives of the contaminants were, on average, 10 days for arsenate, 7 days for chromate, and 2.5 days for copper. The results, averaged over all ports, are shown graphically in Figure 2. To summarize, in 27 days:
- Arsenate was reduced from 26 mg/L to 0.5 mg/L
- Chromate was reduced from 15 mg/L to 0.7 mg/L
- Copper was reduced from 4 mg/L to < 0.1 mg/L
After 27 days of operation, flow was begun to assess the stability of the immobilized metals. Three pore volumes of water exposed to oxygen were flushed through the ASV for 30 days and concentrations of dissolved metals did not increase.
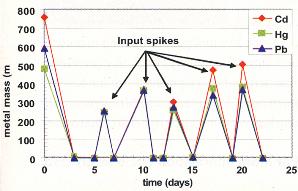
Results for Cadmium, Mercury and Lead
The results for the heavy metal (Cd, Hg and Pb) experiment are presented in Figure 3, which shows that repeated metal input spikes were drastically reduced at all ASV ports 1 to 3 days after injection. In order to show the dramatic decrease in soluble metal concentrations after each input spike, the results in Figure 3 are given as mass of metal (either the mass in an input spike or the average mass of metal in the ASV dissolved phase after an input spike). Concentrations in the 20 mg/L to 100 mg/L range were input and, in 1 to 3 days, decreased to less than 50ìg/L (micrograms per liter) and, in some cases, to less than 10 (10 ìg/L. Metal-contaminated water in volumes greater than the ASV's pore volume of 10 L (liters) were input. All ASV effluent from these inputs had metal concentrations at non-detect levels (<10 ìg/L). Clearly, MRC effectively removed, from solution, large quantities of Cd, Hg and Pb. The ASV was then flushed with three pore volumes of oxygenated tap water with an oxidation reduction potential (ORP) in the 200 mV (millivolt) range. No metals were mobilized by the fresh, oxygenated water.
Stability of Immobilized Metals
A key question concerns the stability of the metals immobilized by MRC. This question must be addressed in two parts, given MRC's two-stage mechanism of metals immobilization. The first stage is complexation by cysteine. The ASV experiments presented here show that, for arsenic, chromium, copper, cadmium, mercury and lead, these complexes are stable when contacted with several pore volumes of oxygenated, pH 5 water at a high seepage velocity (219 feet per year). Based on these results, we are confident that other heavy metals will remain precipitated under most aquifer conditions during this first stage.
The second stage is initiated by biodegradation of the organic portion of the metal-cysteine complex and the creation of a metal sulfide solid. These metal sulfide solids can permanently become part of the soil matrix, if they remain precipitated. Geochemical modeling was used to determine the ORP and pH ranges in which these metal sulfide solids remain precipitated under equilibrium conditions. The geochemical modeling was done with the software package Geochemist's Workbench, a widely-used geochemical modeling code that was developed at the University of Illinois in Champaign, Ill.
Geochemical model simulations show that sulfide solids of most heavy metals and arsenic remain precipitated at pH 6-7 and ORP less then zero. Additionally, the geochemical modeling shows that many metal sulfide solids remain precipitated at positive ORP values as well, depending on the input geochemical conditions. Note that equilibrium geochemical modeling does not address the rate at which dissolution occurs. The kinetics of dissolution may be so slow as to prevent measurable dissolved metal concentrations, even if the ORP and pH of the aquifer indicate that, according to equilibrium geochemical modeling, equilibrium dissolution should occur.9 Thus, the stability of immobilized metals must be assessed based on site-specific geochemistry.
Conclusions
In conclusion, these laboratory studies clearly demonstrate the ability of MRC to immobilize a range of metals that are common groundwater contaminants. MRC is a commercially available product and recently several field injections of MRC have been performed. This advancement in metals and chlorinated solvent remediation is a promising alternative to the more traditional engineering, design and capital-intensive approaches of mechanical extraction and treatment systems. However, caution should be exercised when dealing with any remediation plan that includes in situ metals immobilization. Specifically, the long-term stability of the immobilized metal and the use of risk-based assessments to establish the safety of allowing insoluble metals to remain in the aquifer should be considered.
For more information on MRC and in situ metals remediation contact Regenesis at 949-366-8000 or visit www.regenesis.com.
References
1. U.S. EPA (1995) Record of Decision Information Directory. Office of Emergency and Remedial Response.
2. U.S. EPA (1996) Clean Up of the Nation's Waste Sites: Markets and Technology Trends, 1996 Edition. Solid Waste and Emergency Response. EPA 542-R-96-005, PB 96-178041.
3. U.S. EPA (1994) Analysis of Facility Corrective Action Data. Office of Solid Waste and Emergency Response, Technology Innovation Office.
4. U.S. DOD (1995) Office of the Deputy Under Secretary of Defense (Environmental Security), Restoration Management Information System.
5. U.S. EPA (1997) Recent Developments for In Situ Treatment of Metal Contaminated Soils. Office of Solid Waste and Emergency Response, Technology Innovation Office.
6. Riley, R.G. and J.M. Zachara (1992). Chemical Contaminants on DOE Lands and Selection of Contaminant Mixtures for Subsurface Science Research. DOE/ER 0547; U.S. Department of Energy/OER: Washington, D.C.
7. GWRTAC (1997). Remediation of Metals-Contaminated Soils and Groundwater. Prepared by C.R. Evanko and D.A. Dzombak. Ground Water Remediation Technologies Analysis Center, Pittsburgh. www.gwrtac.org.
8. U.S. EPA (1997b) Technology Alternatives for the Remediation of Soils Contaminated with As, Cd, Cr, Hg and Pb. Office of Emergency and Remedial Response. Office of Research and Development, EPA/540/S-97/500.
9. Stumm, W. and J.J. Morgan (1996). Aquatic Chemistry. John Wiley & Sons Inc. New York.
10. Dvorak, D.H., R.S. Hedlin, H.M. Edenborn, and P.E. McIntire (1992). "Treatment of Metal-Contaminated Water Using Bacterial Sulfate Reduction: Results from Pilot-Scale Reactors." Biotechnology and Bioengineering, vol. 40, no. 5, pgs. 609-616.
11. Bae, W.; W. Chen, A. Mulchandani and R.K. Mehra (2000). "Enhanced Bioaccumulation of Heavy Metals by Bacterial Cells Displaying Synthetic Phytochelatins." Biotechnology and Bioengineering, vol. 70, no. 5, pgs. 518-524.
12. Howard, M. and J.A. Holcombe (2000). "Polyamino Acid Chelation for Metal Reduction." Chapter 12 in I.K. Iskandar (Ed) Environmental Restoration of Metals-Contaminated Soils. CRC Press/Lewis Publishers, New York.
13. Delnomdedieu, M., M.M. Basti, J.D. Otvos, and D.J. Thomas (1994). "Reduction and Binding of Arsenate and Dimethylarsenite by Glutathione: A Magnetic Resonance Study." Chemico-biological Interactions, 90: 139-155.
14. Koenigsberg, S.S. and W.A. Farone (1999). "The Use of Hydrogen Release Compound (HRC®) for CAH Bioremediation." In A. Leeson and B.C. Alleman (Eds), Proceedings of the Fifth International In Situ and On-Site Bioremediation Symposium. 2(5), pgs. 35-42, Battelle Press, Columbus, Ohio. Available at www.regenesis.com.
McGregor, R. (2003) Mitigation and Remediation of Acidic Drainage. Live Internet Seminar SCR-0221, Environmental Institute for Continuing Education (EICE), www.Enviro-Institute.com.
Regenesis (2003) "ASV Studies." Technical Bulletin 2.4.3, www.regenesis.com.
This article originally appeared in the 11/01/2003 issue of Environmental Protection.
About the Authors
Bryan W. Vigue is marketing manager at Regenesis
Anna Willett is research and development manager at Regenesis, which is based in San Clemente, Calif.
Stephen Koenigsberg is vice president of research and development at Regenesis.